The previous article introduced the concept that quantum mechanics is required to describe the behavior of electrons, which in turn drives chemical behaviors such as reactivity and polarization. This article will share more information about the electronic structure of atoms and molecules, and how they affect chemical properties.
As explored in the previous article, the wavefunction provides information about the likelihood of finding an electron in a particular region of space around the nucleus. By solving the Schrödinger equation, one can determine the wavefunction and consequently the probability distribution of an electron’s location around the nucleus, as well as its energy. The regions of space where electrons are most likely to be found are referred to as orbitals.
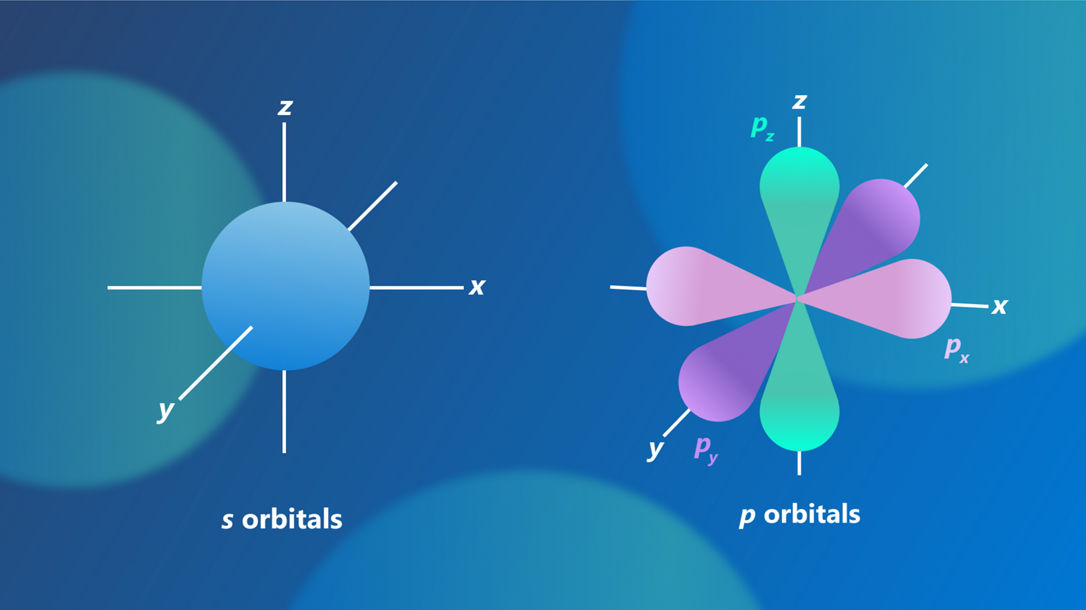
There are four basic types of atomic orbitals, each with a distinctive shape. They are usually labelled s, p, d and f. The simplest type of atomic orbital is the s orbital, which has a spherical shape centered around the nucleus. It is like a fuzzy sphere with the nucleus at its center which describes where the electron can be found with the highest probability. The p orbitals look like two balloons tied together with the knot centered on the nucleus. Each “balloon” is called a lobe. There are three kinds of p orbitals, oriented along the x, y, and z axes in space. d and f orbitals have different shapes as well, but they are more complex.
An atom with many electrons will have multiple atomic orbitals with electron distributions that move progressively further away from the nucleus. Each one of these atomic orbitals has a specific energy level associated with it, and the electrons belonging to each orbital possess that amount of energy. Electrons can absorb energy and be promoted to higher energy levels, in a process known as excitation. For an electron to transition from a lower energy level to a higher one, the energy absorbed must exactly match the energy difference between the initial and final energy levels.
Molecular orbitals
When atoms combine to form molecules, their atomic orbitals combine to form molecular orbitals. In molecular orbitals, electrons are no longer confined to a single atom but are shared between multiple atoms in the molecule – this is what binds the molecule together.
The process of forming molecular orbitals involves the constructive and destructive interference of atomic orbitals. When atomic orbitals combine constructively, a bonding molecular orbital is formed. This bonding orbital has a lower energy compared to the original atomic orbitals, thus increasing the stability of the molecule. On the other hand, when atomic orbitals combine destructively an antibonding molecular orbital is created, which has higher energy and reduces the stability of the molecule.
Molecular orbitals exhibit a variety of shapes and arrangements, which depend on the types of atomic orbitals that combined to form them. They can be spread over the entire molecule or concentrated between specific atoms. These molecular orbitals define the electronic structure of the molecule and influence its chemical properties, such as reactivity, color and polarization.
Understanding atomic and molecular orbitals provides insights into how atoms bond together, why certain molecules are stable, and how they interact with light and other molecules. This knowledge is fundamental to fields such as chemistry, materials science, and biology. Try Copilot in Microsoft Quantum to learn more about molecular orbitals in an interactive way today.