Classical physics, which describes the motion of objects on a macroscopic scale, fails to explain the behavior of electrons and other particles that exist on atomic and subatomic scales. Instead, quantum mechanics must be applied to describe these behaviors. One of the central tenets of quantum mechanics is that particles exhibit both particle-like and wave-like properties – a concept known as wave-particle duality. This wave-particle duality leads to a number of curious behaviors such as the principles of superposition and entanglement, which are exploited by quantum computers to perform operations impossible to achieve on a classical computer.
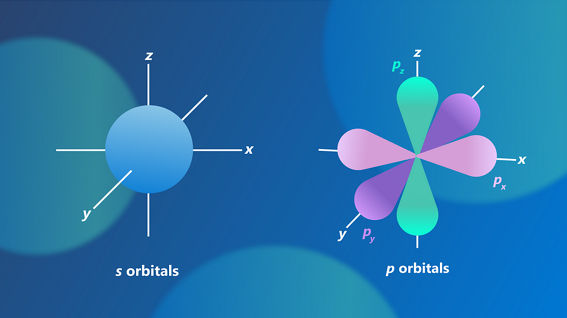
The principle of superposition is particularly important in the context of quantum chemistry. Unlike classical physics, where particles have well-defined positions, electrons in atoms are described by a probability distribution known as a wavefunction. The wavefunction provides information about the likelihood of finding an electron in a particular region of space around the nucleus, as well as its energy.
By solving the Schrödinger equation, which is the fundamental equation of quantum mechanics, one can determine the wavefunction and consequently the probability distribution of an electron’s location around the nucleus. The regions of space where electrons are most likely to be found are referred to as orbitals. Quantum chemistry aims to apply this knowledge to explain and predict chemical behaviors and properties. More information on orbitals can be found here.
Understanding chemical behavior
An accurate understanding of the electronic structure of matter is essential for explaining chemical properties and behavior. Quantum chemistry makes it possible to understand why certain compounds are stable, how molecules absorb and emit light, and even how drugs interact with biological systems.
By solving the Schrödinger equation, quantum chemists can explain and even predict these properties. Unfortunately, it is impossible to exactly solve the Schrödinger equation using classical methods for systems beyond just one or two atoms. Instead, approximations are used to model the behavior of these systems using powerful computers today.
Through advanced computational methods and experimental techniques, quantum chemists are continuously uncovering the secrets of matter at the quantum level. Their findings provide the foundation for advancements in various fields, including materials science, drug design, and energy research. The invention of a quantum supercomputer could enable scientists to understand and predict chemical behaviors and properties with much greater accuracy, paving the way for humanity to solve world-impacting problems such as global warming and food insecurity. Try Copilot in Microsoft Quantum to learn more about quantum chemistry today.